Our Pipeline: First-In-Class
Bicara is developing bifunctional therapies that precisely target solid tumors and deliver a tumor-modulating payload to the tumor site, with our lead program advancing in clinical development.
- PROGRAMTARGETDISCOVERYIND-ENABLINGPHASE 1PHASE 2PHASE 3Indication
- BCA101EGFR / TGF-βEGFR / TGF-βcSCCHNSCC, SCAC, SqNSCLC
- Discovery ProjectsUndisclosedUndisclosedSolid Tumors
We seek out a powerful biological rationale and strong potential to address significant unmet needs, which include patients who are refractory to immune checkpoint inhibitors and first-line targeted therapies.
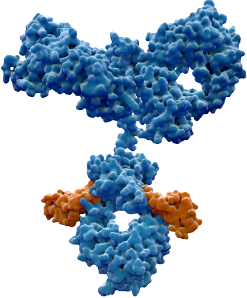
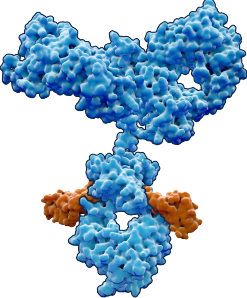
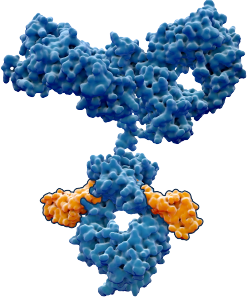
Our Lead Program: BCA101
First-in-class EGFR / TGFβ-trap bifunctional antibody
Epidermal growth factor receptor (EGFR), a protein expressed on the surface of several tumor types, is one of the most extensively validated tumor-associated antigens.
Monoclonal antibodies directed at EGFR suppress the proliferation of EGFR-driven tumor cells and restrict tumor growth and metastasis. Inhibiting EGFR alone is inadequate to achieve durable therapeutic responses. Our solution: Simultaneous inhibition of EGFR and TGF-β — a signaling molecule that promotes tumor growth in the presence of EGFR and that plays a key role in suppressing the immune response in the tumor microenvironment.
Our lead program is thus a dual-action bifunctional antibody that both inhibits EGFR and disables TGF-β directly at the site of the tumor. With this approach, we hope to achieve superior anti-tumor efficacy with an improved therapeutic window.
Phase 1/2 Clinical Study
We have completed dose escalation studies evaluating BCA101 as a single-agent and in combination with pembrolizumab, and have initiated the dose expansion arm of our clinical study.
BCA101 will be administered in combination with pembrolizumab as first-line therapy in patients with unresectable, recurrent or metastatic HNSCC and in other solid tumors.
For more information, please visit clinicaltrials.gov.
Enrolling trial sites

Houston, TX

New York, NY

Boston, MA

Toronto, ON

New York, NY

Pittsburgh, PA

Charleston, SC

Los Angeles, CA

San Diego, CA

Tampa, FL

Cleveland, OH

Providence, RI